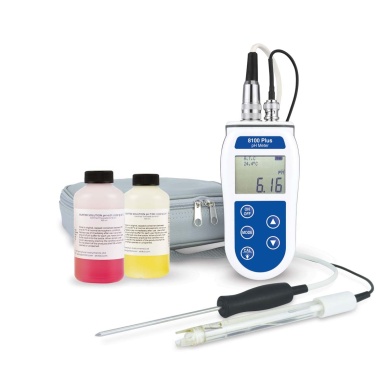
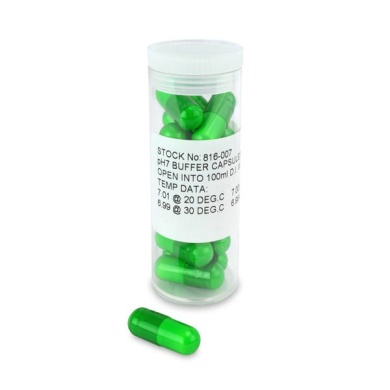
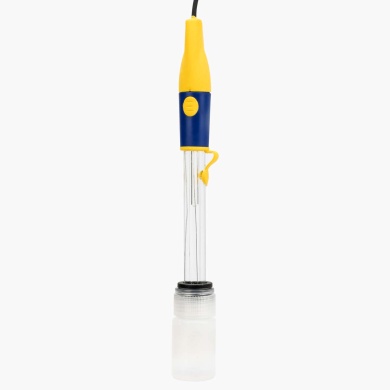
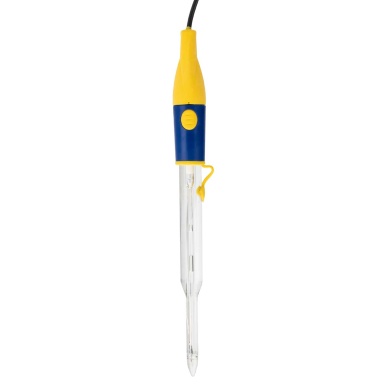
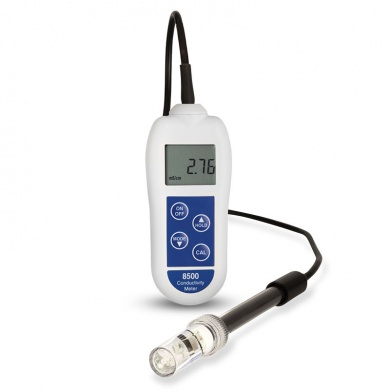
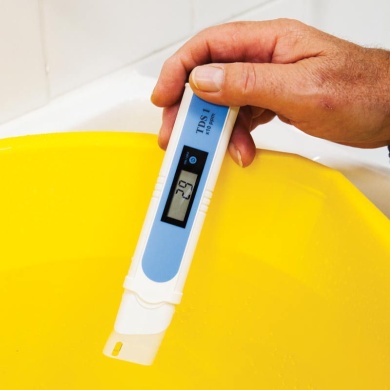
£144.00 inc VAT
£28.80 inc VAT
£12.00 inc VAT
£40.20 inc VAT
£40.20 inc VAT
£180.00 inc VAT
£247.80 inc VAT
£164.40 inc VAT
£96.00 inc VAT
£80.40 inc VAT
£25.20 inc VAT
£126.00 inc VAT
£126.00 inc VAT
£57.60 inc VAT
£144.00 inc VAT
£82.80 inc VAT
£92.40 inc VAT
£12.60 inc VAT
£13.20 inc VAT